EORTC is an academic, independent, non-profit organization under Belgian law, with no direct government subsidy. Our revenue comes from grants from institutional, corporate and private donors and fees charged for studies conducted with partners or services to the oncology community that enables us to fulfil our mission and vision.
Clinical studies evaluating new drugs for potential registration, or testing therapeutic agents, are conducted in partnership with commercial organizations. This funding is subject to EORTC’s strict principles of independence. The pie chart shows the total income of EORTC and its source, the figures provided refer to the book-year 2022:
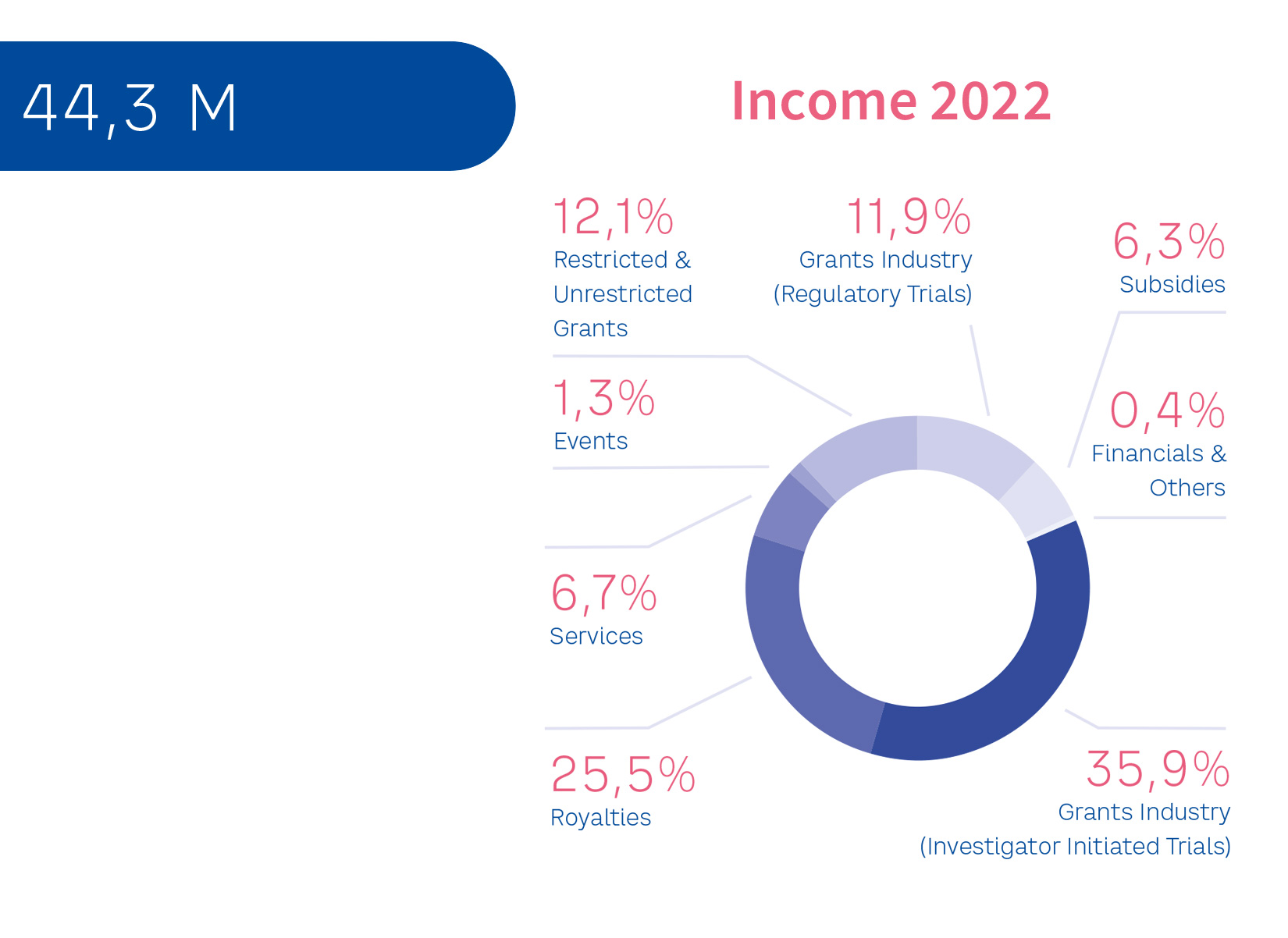
Legend
Grant Industry (Regulatory Trials): Income generated through partnership with Pharmaceutical industry for clinical trials aimed at supporting licensing application
Grant Industry (Investigator Initiated Trials): income generated following grant application to Pharmaceutical Industry for clinical trials aimed at improving practice
Restricted & unrestricted grants: support obtained through public and private non-for profit organizations (such as but not limited to European Grants, national cancer league grants, private or corporate donations,…)
Royalties: income generated for clinical research expert services
Services: revenue related to the translation of Quality of Life questionnaires
Subsidies: eligible public support for non-for profit research organisations.
Overall, the highest cumulative contribution by a single pharmaceutical company does not exceed 22% of the total revenues; the average is less than 3.2%.
EU projects
The European Union supports projects with great public health value, providing funding that is not otherwise available. Over the years, EORTC has participated in numerous projects funded by the European Commission in various cancer and clinical research-related fields. The EU funding initiatives that have supported our research efforts are:


Horizon 2020
Funding from Industry
Listed below are the companies currently involved in EORTC clinical trials.
- ABBVIE
- AGENDIA
- ALPHA TAU MEDICAL
- AMGEN
- ASTELLAS
- ASTRAZENECA
- BAYER
- BEIGENE
- BLUEFISH PHARMACEUTICALS
- BOEHRINGER INGELHEIM
- BRISTOL-MYERS SQUIBB
- CELLDEX THERAPEUTICS
- COTHERA BIOSCIENCES
- ELI LILLY & CO.
- EXELIXIS
- ROCHE
- FERRING PHARMACEUTICALS
- GLAXOSMITHKLINE
- HELSINN HEALTHCARE
- IMMUNOCORE
- INCYTE
- INNATE PHARMA
- JOHNSON & JOHNSON
- KYOWA KIRIN
- MENARINI
- MERCK
- MSD
- NH THERAGUIX
- NOVARTIS
- PFIZER
- PHARMA MAR
- PIERRE FABRE
- RECORDATI
- SANOFI-AVENTIS
- SEAGEN
- TAKEDA
- TRIACT THERAËUTICS
EORTC Cancer Research Fund (ECRF)
The EORTC Cancer Research Fund (ECRF) is an independent non-profit association founded in 1976 under the honorary presidency of His Royal Highness Prince Philip, the Duke of Edinburgh. ECRF’s objective is to promote, encourage and support research on the treatment of cancer. To fulfil this, it engages in financing studies and research performed by the international non-profit organisation, EORTC.
Over the past four decades, the ECRF has raised millions of euros to fund EORTC trials and projects to develop new treatments and care, which have greatly increased survival and quality of life of patients with cancer.
Funds come from grants and donations from institutional, corporate partnerships, private and public foundations, individual private donors, legacies and organisations’ in financial or in-kind donations to EORTC’s activities.
Every single donation goes in full to specific EORTC projects chosen either by the donor or in the case of an unrestricted gift, by the EORTC to cancer research that lacks funding. In both cases, donors are able to follow and be part of EORTC’s journey in increasing survival and quality of life for all cancer patients.
ECRF is privileged to count HRH Princess Dina Mired as its Honorary President and Count Diego du Monceau de Bergendal as the Chairman of the Fund.
Public Foundations and Grantors
For an overview of our funders please see:
https://www.eortcresearchfund.org/funding/funding-partners-supporters/
EORTC is grateful to all its supporters including many private individuals who over the years have offered their contributions so that we can ensure therapeutic progress for all cancer patients.

