Q&A: A missing conversation on the price of cancer drugs
13 May 2016
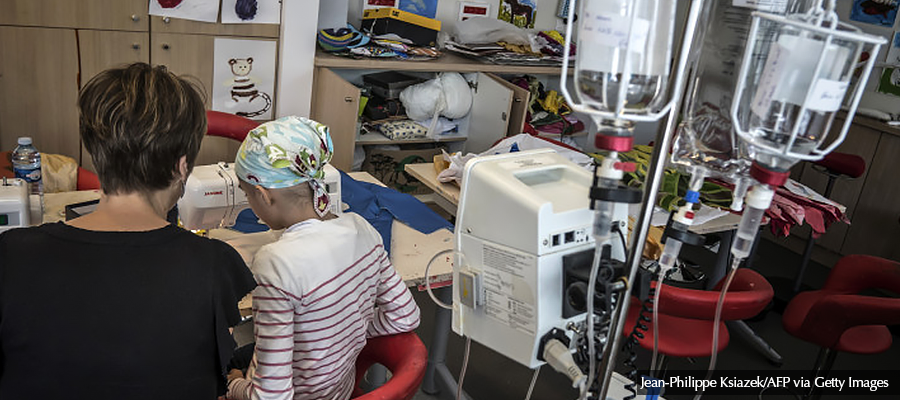
A novel approach to judge which new cancer drugs are worth reimbursing is emerging within the European Organisation for Research and Treatment of Cancer, an independent research group. Denis Lacombe, its director general, says the growing understanding of tumor biology can equip European authorities to make more informed decisions based on performance in real-life conditions. In an interview with POLITICO, he suggested how to ease access to the evidence that could radically improve decision-making.
The read the full article on the Politico Pro website.
Related News
Meet the new EORTC Board
9 Jul 2024
We are pleased to announce the release of the EORTC 2023 Annual Report
17 Jun 2024
Dr Denis Lacombe, EORTC CEO, appointed stakeholder co-chair of ACT EU advisory group
24 May 2024
Clinical Trials Day 2024: a Q&A on pragmatic clinical trials
20 May 2024
EORTC/EMA workshop suggests an international way forward for treatment optimisation studies
8 May 2024
EORTC’s Participation at the ESTRO Congress 2024
29 Apr 2024
EORTC: Advancing research and treatment for rare cancers
29 Feb 2024
EORTC Fellowship Programme: celebrating more than 20 years of impactful collaboration
22 Feb 2024
Appointment of Malte Peters as EORTC Strategic Alliance Officer
9 Feb 2024
Unique series of workshops in partnership with the European Medicines Agency (EMA)
7 Feb 2024